Although only developed within the last hundred years, polymers have become an essential part of our everyday lives. We use them for clothing, carpets, plastics, cooking, construction, and even for fighting fires. We produce over 100 million tons of synthetic polymers annually, and these materials account for 7 % of our municipal refuse.1,2 Because of their industrial value, polymers have been extensively studied. The number of research papers in the polymer literature far exceeds what a single person could read in an entire lifetime.
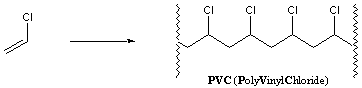
The word "polymer" is derived from the Greek word "poly," meaning many, and the Greek word "mer," meaning unit. Polymers are very large molecules composed of many repeating units. These units are called monomers ("mono" means one) and are usually very small molecules. Polymers of extremely long chain lengths have important macroscopic characteristics. PVC, for example, is composed of thousands of vinylchloride monomer units:

PVC is a hard, rigid material used for pipes and phonograph records. Addition of plasticizers into the polymer affords a softer material that is used for raincoats, shower curtains and garden hoses. Another polymer, Orlon, is composed of repeating acrylonitrile monomer units:
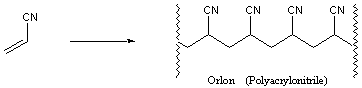
We fashion Orlon into fibers for use in clothing and carpets. Teflon is also a synthetic polymer, composed of repeating tetrafluoroethylene subunits:
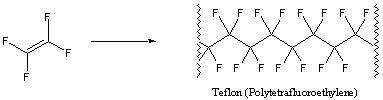
Due to its low coefficient of friction, we use Teflon for lining pots and pans. PVC, Orlon, and Teflon are only a few examples of the many polymers that we use in our everyday lives.
Nature also produces polymers, such as starch (as in potatoes) and cellulose (in trees and cotton):

While nature recycles these polymers, it often has trouble recycling our synthetic polymers. For this reason, there has been extensive research in the area of biodegradable-plastics. While biodegradability is a desirable characteristic, photodegradability is usually not. Degradation by ultraviolet light severely limits a polymerís application. Unstabilized PVC, for example, discolors and deteriorates after several weeks of natural weathering.3 Stabilization technology has rendered PVC a major plastic material today.4
Photodegradation generally occurs either through Norrish reactions or through photooxidation.5 There are two major types of Norrish reactions, Type I and Type II.
Norrish Type I reactions involve cleavage of a bond to a carbonyl group:6
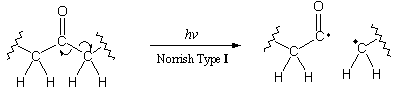
The free radicals formed in this process can respond in many ways. If the radicals recombine to form a bond, then no net change occurs. If the radicals respond differently, then the process ruptures a bond. These radicals also initiate chemical reactions that further sever the polymer chain.
Norrish Type II reactions involve carbonyl abstraction of a hydrogen atom:6
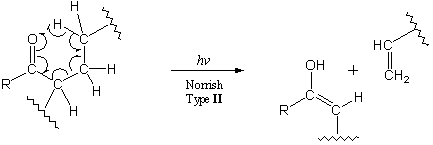
This reaction parallels McLafferty rearrangements of radical cations in mass spectrometry. In Norrish Type II photochemistry, the carbonyl group need not be part of the polymer backbone for photodegradation to take place.
Polymers can gain carbonyl groups through reactions with trapped impurities or, more importantly, through photooxidation reactions. The role of photooxidation in photodegradation is not entirely known, but it is believed7 that reactions of polymers with molecular oxygen (O2) produce hydroperoxides. These hydroperoxides can lead to the introduction of carbonyl groups in the following manner:
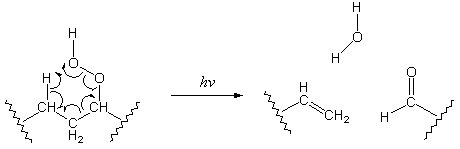
Reactions of this kind degrade the polymer chain and create carbonyl groups that help further degrade the polymer through Norrish photochemistry.
Unstabilized polymers are generally photodegradable. Many additives, called stabilizers, slow down the degradation process substantially by absorbing the sunís damaging ultraviolet light and/or dissipating the energy as heat.8 Stabilizers are generally very complex organic compounds, and their presence often effects the physical properties of polymers.
We have produced polymers that photodegrade very slowly, and we have produced polymers that photodegrade in just half a day under sunlight.9 We have gained control over polymer photodegradation through an understanding of the underlying chemistry.
For more information on polymers, see the following website: http://www.psrc.usm.edu/macrog/index.htm
References